Results
with
DUPIXENT
Actual DUPIXENT patient.
Individual results may vary.
results you can
see and feel
Backed by science, DUPIXENT is the first FDA-approved treatment for adults with prurigo nodularis (PN).
CLINICAL TRIAL RESULTS
In two clinical studies with 311 adults with PN:
LESS ITCH
59% on DUPIXENT vs 19% not on DUPIXENT
CLEAR OF PN BUMPS
46% on DUPIXENT vs 17% not on DUPIXENT
CLEAR OF PN BUMPS
46% on DUPIXENT vs 17% not on DUPIXENT
LESS ITCH
59% on DUPIXENT vs 19% not on DUPIXENT
IN ONE STUDY
LESS ITCH
37% on DUPIXENT vs 22% not on DUPIXENT
VIEW SAFETY RESULTS
MOST COMMON SIDE EFFECTS IN PATIENTS WITH PRURIGO NODULARIS INCLUDE:
- Eye and eyelid inflammation, including redness,
swelling, and itching, sometimes with blurred vision - Herpes virus infections
- Common cold symptoms (nasopharyngitis)
- Dizziness
- Muscle pain
- Diarrhea
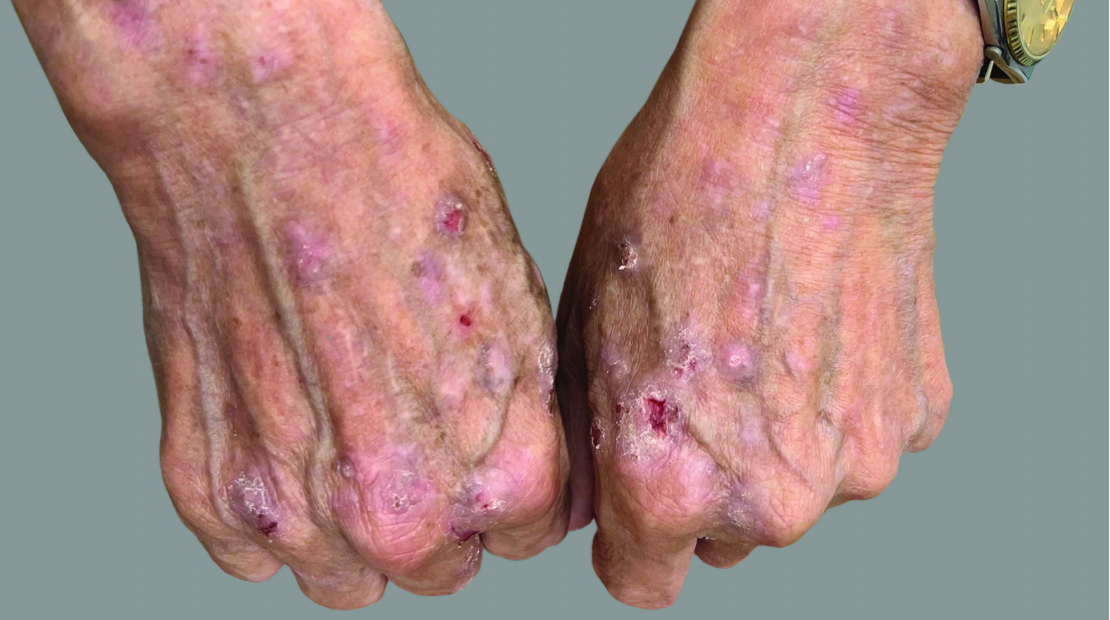
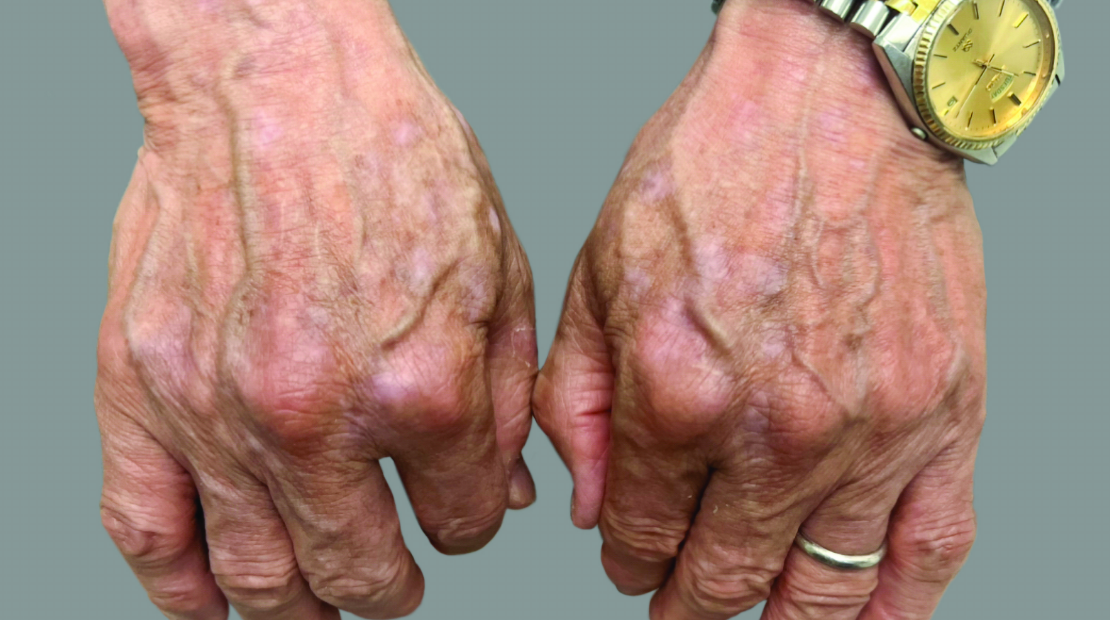
View Before and After Results With DUPIXENT
Results with 32 weeks of treatment with DUPIXENT
to see results
to see results
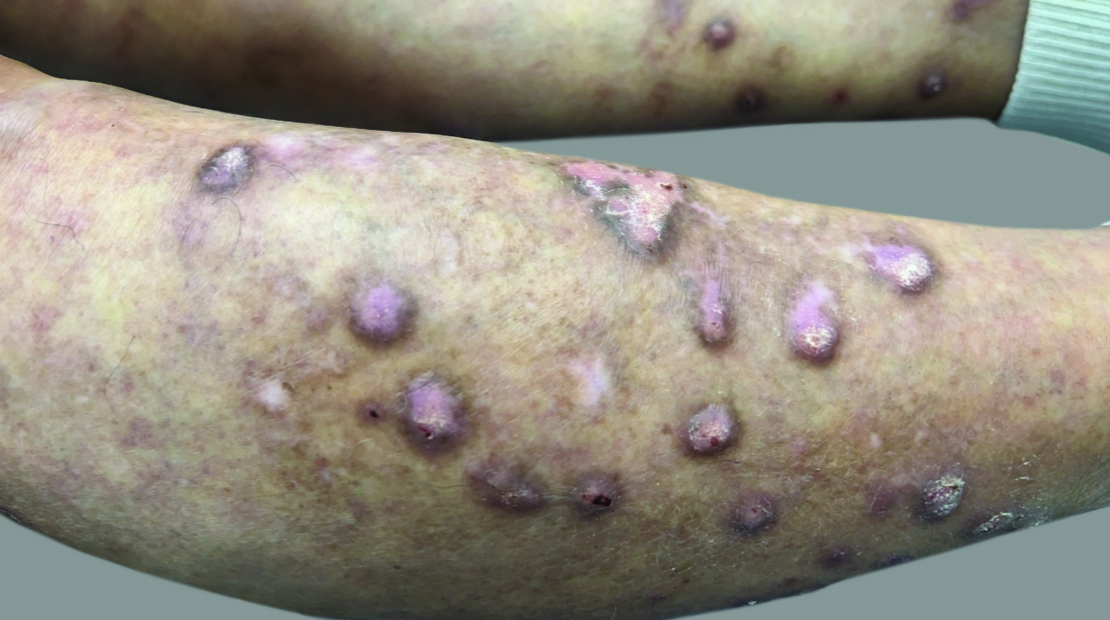
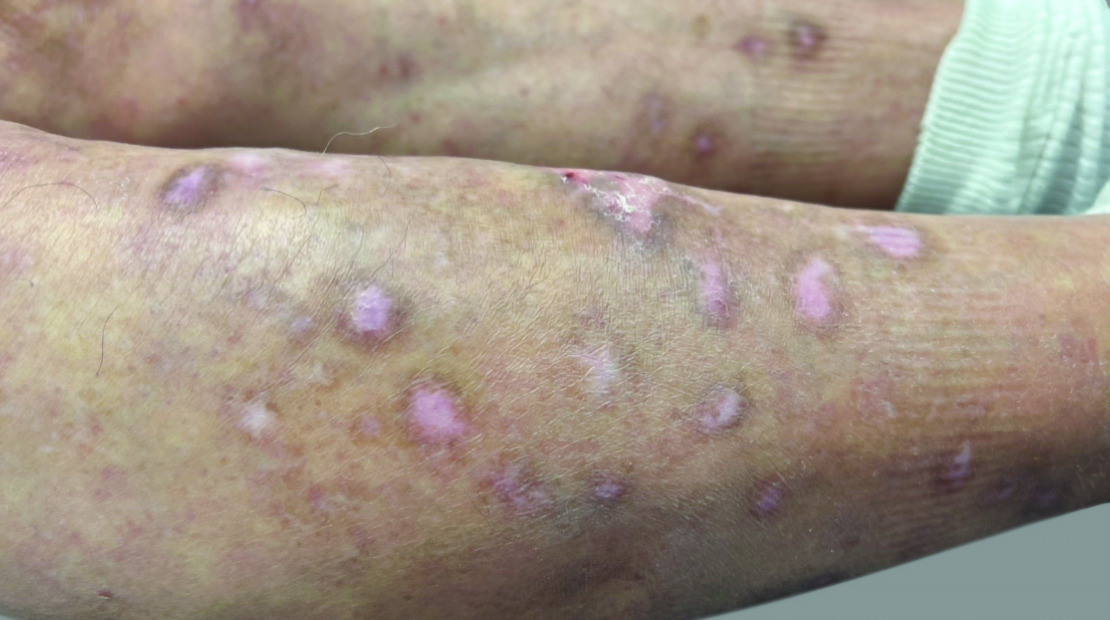
Actual patient shown. Additional factors, including possible use of other treatments, may have impacted results. Individual results may vary.
A Helpful Reminder
Get valuable info from this video to help you during your treatment journey.


